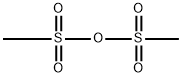
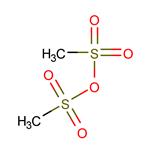
Methanesulfonic anhydride:Application and Chemical Studies
GeneraL Description
Methanesulfonic anhydride with the form of the white solid as an anhydride of methanesulfonic acid , has been used as an excellent reagent for the improvement in Friedel-Crafts acylation reaction. Methanesulfonic anhydride is readily available, inexpensive, eco-friendly, bench stable, non-hygroscopic reagent and easy to handle.[1]

Figure 1 Solution of Methanesulfonic anhydride
Preparation
Methanesulfonic acid (23.6mL, 0.25 mol) was dissolved in thionyl chloride (100 mL, 1.37 mol) and the mixture was heated to reflux for 0.5 h. NOTE: It is not necessary to conduct this reaction under argon since HC1 gas is constantly evolved, blanketing the reaction mixture. Excess thionyl chloride was removed by distillation. The resulting light yellow solid was recrystallized from anhydrous diethyl ether (18.84g,44%). It was stored in a desiccator over Drierite at 5°C. Under these conditions, mesic anhydride will last 2-3 weeks, but even slight decomposition warrants discarding the material.[2]
The synthesis of methanesulfonic anhydride from the corresponding acid with thionyl chloride and the purificaton of the product from recrystallization solvents were studied. The results showed that the yield of product can be up to 90% under optimum conditions: the reaction temperature is 80℃, the molar ratio of methanesulfonic acid to thionyl chloride is 1/2.5, the reaction time is 4 hours, the time of evaporation of excessive thionyl chloride is 1 hour, and the solvent is benzene isopropyl ether (vol. ration is 1:0.7~1).[3](MeSO2)2O was prepd. in 95% yield by mixing MeSO3H with P2O5 at 0° and distg. under reduced pressure.[8]A combination of absorption bands can be due to the fact that at a concentration of more than 15 M, methanesulfonic acid molecules, by analogy with concentrated sulfuric acid, are capable of forming methanesulfonic anhydride.[12]
Application
One side, the utility of methanesulfonic anhydride for promoting the Friedel-Crafts acylation reaction of aryl and alkyl carboxylic acids. This reagent allows the preparation of aryl ketones in good yield with minimal waste containing no metallic or halogenated components.[4]Methanesulfonic anhydride plays an important role in the direct synthesis of thioester in light of study. This metal-, halogen- and solvent-free approach for the synthesis of thioesters will generate broad applications among practitioners of synthetic, pharmaceutical and industrial chemistry.[1]On the other hand, regioselective methanesulfonylation of simple aromatics using methanesulfonic anhydride can be achieved over zeolite catalysts. For example, methanesulfonylation of toluene over various cation-exchanged zeolite β catalysts affords higher para-selectivity in the synthesis of methyl tolyl sulfone than standard Friedel–Crafts methanesulfonylation utilising aluminium chloride.[5]Using methanesulfonic anhydride can control composition of the methanesulfonic acid by combining NMR, conductimetry, viscometry, and differential refractometry.[6]
Methanesulfonic anhydride can be used as as the mesylating reagent avoids the production of any halide waste in newly developed two-step sequential mesylation/arylation.[7]The reaction of methanesulfonic anhydride with toluene over various cation exchanged zeolite beta catalysts affords higher para-selectivity and better yields of Me tolyl sulfone than std.[9]The methanesulfonic anhydride can be used in anisole for converting nitroanisole.The effect of methanesulfonic anhydride is to react with the nitrate ion to produce methanesulfonyl nitrate. This reactive intermediate then reacts with the aromatic ring to produce the corresponding nitro aromatic compounds.The methanesulfonic anhydride reacted with this weakly associated ion of nitrate anion to produce methanesulfonyl nitrate and that for potassium and ammonium nitrate would not work.Methanesulfonic anhydride (1 mmol, 0.17g) in a mortar with a mixture of 1,4-dimethoxybezene (1 mmol, 0.14g), reagent (1) (1 mmol, 0.42g) was employed for nitration of 1,4-dimethoxybezene with other chemical compoundto 2-Nitro-1,4-dimethoxybenzene.[10]In order to improve the reaction efficiency and operation, solid methanesulfonic anhydride was used as the reaction reagent in a very weak acidic solvent.[11]
Reference
[1]Singh P, Peddinti RK. Methanesulfonic anhydride-promoted sustainable synthesis of thioesters from feedstock acids and thiols[J]. Journal of Chemical Sciences, 2021, 133: 1-12.
[2]Jones G. S.Synthesis and reactivity of oxyglycals.[J]Available from ProQuest Dissertations Theses Global.1992.
[3]Wei D W, Sheng L F, Yin J Z, et al. Study on the Synthesis and Purification of Methanesulfonic Anhydride[J]. Chemical Reaction Engineering and Technology, 2003.
[4]Wilkinson MC.“Greener” Friedel?Crafts Acylations: A Metal-and Halogen-Free Methodology[J]. Organic Letters, 2011, 13(9): 2232-2235.
[5]Smith K, Ewart GM, El-Hiti GA, et al. Study of regioselective methanesulfonylation of simple aromatics with methanesulfonic anhydride in the presence of zeolite catalysts[J]. Organic biomolecular chemistry, 2004, 2(21): 3150-3154.
[6]Roitman DB, McAlister J, Oaks FL. Composition characterization of methanesulfonic acid[J]. Journal of Chemical and Engineering Data, 1994, 39(1): 56-60.
[7]Ferguson DM, Rudolph SR, Kalyani D. Palladium-catalyzed intra-and intermolecular C–H arylation using mesylates: synthetic scope and mechanistic studies[J]. ACS catalysis, 2014, 4(7): 2395-2401.
[8]Paul RC,et al. Methanesulfonic anhydride.[J].Chemistry Industry. 1971.
[9]Smith K,et al. Regioselective methanesulfonylation of toluene catalysed by cation-exchanged zeolite β[J]. Journal of the Chemical Society, Perkin Transactions 1, 1997 (8): 1085-1086.
[10]Hajipour AR, Ruoho AE. A fast and mild method for nitration of aromatic rings[J]. Phosphorus, Sulfur, and Silicon, 2004, 179(2):221-226.
[11]Zhao R, Lu W. Palladium-Catalyzed β-Mesylation of Simple Amide via Primary sp3 C–H Activation[J]. Organic letters, 2017, 19(7):1768-1771.
[12]Akhmedov MA, Khidirov SS, Khibiev KS. Modification of cellulose in the solution of methanesulfonic acid[J]. Russian Chemical Bulletin, 2021, 70:412-419.
Related articles And Qustion
Lastest Price from Methanesulfonic anhydride manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 7143-01-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons
US $10.00/kg2025-04-21
- CAS:
- 7143-01-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt